






 |
The drawings in this collection are taken from the doctoral research of Daniel C. Coy (Chemical Engineering Department, Iowa State University, 1993). The various surfaces depict 3-dimensional, parametric sections of thermodynamic fundamental and state functions for pure, binary, and ternary systems representing mixtures of ethylene, normal butane, and carbon dioxide in the fluid phases.
Fundamental functions portray variable relationships that yield complete sets of thermodynamic information. The various combinations U-V-S-N, H-P-S-N, A-T-V-N,... for pure materials; A-N1-V-T-N, G´ -T-P-µ1-N2, ... for binary systems, etc., are coupled mathematically through the Legendre transform – a mapping that links the coordinate systems while preserving thermodynamic data. These functions were first noted by Massieu (1869), who referred to them as "characteristic," but their crucial and practical importance in thermodynamics was stated by J. W. Gibbs in his second and third papers (1873, 1875-1878).
Fundamental functions contain at least one nonmeasurable or "derived" thermodynamic property and therefore cannot be constructed directly from experimental data. They are developed from integrations of real measurements, often cast in the form of pressure-volume-temperature-composition relationships (called equations of state) and heat-capacity correlations. Once constructed they yield their constituent functions through partial differentiation. Several equation-of-state models are shown in this collection — P-V-T-N, T-S-P-N,... for pure materials;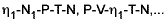 for binaries, etc. for binaries, etc.
Gibbs was the first to suggest that these functions could be interpreted quantitatively from their geometric analogies. There is a regularity among the Gibbs models that reflects the universal character of thermodynamic functions and the nature of the information transformation that couples them. Understanding the models in depth requires an appreciation of thermodynamic stability theory, particularly as it influences the slopes and curvatures of various portions of each surface.
In this collection the models appear as geometric objects — surfaces in various coordinate systems that show qualitatively how thermodynamic functions — energy, enthalpy, Helmholtz and Gibbs energies, and others without common names — vary over the ranges of their natural variables that span the fluid-phase critical region. Color denotes the level of stability shown by certain ranges of properties: blue represents stable states that can be produced experimentally, red shows conditions that are unstable and cannot be produced (i.e., states whose very existence would violate the Second Law and can be understood only in the mathematical sense), and yellow denotes the in-between property ranges termed "metastable" — states that can be produced in the laboratory but that will ultimately revert to more stable conditions.
The interested viewer is referred to Dr. Coy's dissertation, “Visualizing thermodynamic stability and phase-equilibrium through computer graphics” (available from Iowa State University through interlibrary loan) or to one of the excellent textbooks on theoretical classical thermodynamics: Callen, Thermodynamics and an introduction to thermostatistics (1985), chapters 2-6,8; Model and Tester (Reid), Thermodynamics and its applications (1997), chapters 5-7. The Legendre transform and its uses in chemical thermodynamics are discussed in detail by Robert Alberty in Chemical Reviews, Vol. 94, No. 6, Sept/Oct 1994, p. 1457.
The visualizations shown here were produced using MOVIE BYU rendering software. The Peng-Robinson equation of state in its pure and also in its multicomponent form using common mixing rules was taken as the basis for the integrations.
These images and Dr. Coy’s work in general are believed to comprise the first comprehensive analysis and visualization of thermodynamic fundamental functions. Because these relationships are almost always hyperdimensional, computer visualization is a crucial tool for understanding the geometric regularities that exist among them and that result from the use of the Legendre transform.
|