|
The Helmholtz energy-volume-composition surface for a binary mixture of ethylene (component 1) and normal butane (component 2) as modeled by the Peng-Robinson equation in mixture form. The basic variable set is A(T,V,N1,N) scaled to
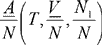
and written simply as A (T, V, x1). As with the pure-fluid models, the property range spans the critical region. Temperature is fixed at the value for which the critical composition is 67 mole-percent ethylene. Colors represent the same stability conditions as before.
Because this is a first transform in a binary system, the geometry (slopes, curvatures, etc.) is the same as for the base function of a pure system (e.g., the UVS model). Thus Gibbs’ rolling plane outlines a succession of pairs of liquid and vapor mixtures in equilibrium – here a vapor phase (green cross) approximately 60 mole % ethylene and a liquid (red cross) approximately 15% ethylene. All points on the surface are at the same temperature; the two slopes of the plane correspond to pressure and to ethylene chemical potential, and its intercept on the back wall (at x1 = 0, thus pure nC4H10, but not shown here) gives the chemical potential of butane. The four phase-equilibrium criteria T,P, µC2, µnC4 are thus satisfied by the states at the red and green tangent points. Note that the plane’s rolling motion follows the directions northeast (toward lower pressures) or southwest (toward higher pressures and the critical point).
|